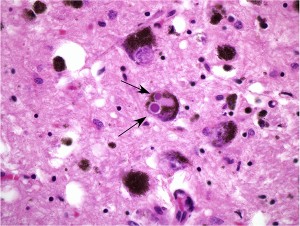
Brain tissue from a patient with Parkinson’s disease. The arrows indicate two Lewy bodies, which contain the pathologic protein alpha-synuclein, within a dopaminergic neuron.
The Hanna Lab studies how cells destroy their own proteins. Protein degradation is crucial for two reasons. First, misfolded proteins may be toxic to cells and must be rapidly identified and destroyed. Failure to destroy toxic misfolded proteins is the underlying basis for many diseases, including most neurodegenerative diseases, including Parkinson’s, Huntington’s, and ALS. Second, by precisely controlling the levels of key regulatory proteins, protein degradation contributes to nearly every cellular process, including the cell cycle, DNA repair, protein transport, and many others.
The ubiquitin-proteasome system is the major pathway for intracellular protein degradation. Substrates fated for destruction in this pathway are labeled with the small protein ubiquitin. This ubiquitin tag then directs the substrates to an elaborate molecular machine known as the proteasome which destroys the target protein while releasing the ubiquitin tag.
Like other large multisubunit complexes, the proteasome is too big to assemble spontaneously. Rather, it is built through an ordered multistep pathway which is coordinated by a family of dedicated molecular chaperones. Shown below is a cryo-EM structure of the 13S proteasome core particle assembly intermediate (3.6 A). This is the first high resolution structure of a proteasome assembly intermediate. A major focus of the Hanna lab is the biochemical and structural analysis of proteasome biogenesis.
We are currently recruiting. Click here for more information.
Comments: 0